Abstract
Acute lymphoblastic leukemia (ALL) is a cancer of the white blood cells and is typically well treated with combination chemotherapy, with a remission state after 5 years of 94% in children and 30-40% in adults. To establish how aggressive the disease is, further chromosome testing is required to determine whether the cancer is myeloblastic and involves neutrophils, eosinophils or basophils, or lymphoblastic involving B or T lymphocytes. This case study is on a 14-year-old patient diagnosed with a very aggressive form of ALL (positive for the Philadelphia chromosome mutation). A standard bone marrow transplant, aggressive chemotherapy and radiation therapy were revoked, with treatment being deemed a failure after 34 months. Without any other solutions provided by conventional approaches aside from palliation, the family administered cannabinoid extracts orally to the patient. Cannabinoid resin extract is used as an effective treatment for ALL with a positive Philadelphia chromosome mutation and indications of dose-dependent disease control. The clinical observation in this study revealed a rapid dose-dependent correlation.
© 2013 S. Karger AG, Basel
Presentation of the Case
A 14-year-old female, P.K., presented with symptoms of weakness, shortness of breath and bruising when she was taken to the Hospital for Sick Children, Toronto, Canada, on the 10th March 2006. She was diagnosed with acute lymphoblastic leukemia (ALL), with >300,000 blast cells present. Acute chemotherapy followed by a standard chemotherapy regimen went on for 6 months after the diagnosis. Upon further analysis, she was found to be positive for the Philadelphia chromosome mutation. A mutation in the Philadelphia chromosome is a much more aggressive form of ALL. When standard treatment options were unsuccessful, a bone marrow transplant was pursued. She successfully received the transplant in August 2006 and was able to be released from isolation 45 days later. She was observed posttransplant by following the presence of blast cells, noted 6 months after treatment. Consequently, in February 2007, aggressive chemotherapy procedures (AALL0031) were administered along with a tyrosine kinase inhibitor, imatinib mesylate (Gleevac), 500 mg orally twice a day. In November 2007, 9 months after the transplant, the presence of premature blast cells was observed and it was determined that another bone marrow transplant would not be effective. In February 2008, in an effort to sustain the patient, another tyrosine kinase inhibitor, disatinib (Sprycel), was administered at 78 mg twice a day with no additional rounds of chemotherapy. The patient experienced increased migraine-like headaches in June 2008. After conducting a CT scan of the head in July 2008, cerebellitis was noted. It was assumed by the primary oncologist that the blast cells could have infiltrated the CNS and be present in the brain, although none were noted in the blood. By October 2008, ten treatments of radiation therapy had been administered to the brain.
On the 4th February 2009, blood was noted in the patient’s stools and a blood cell count revealed the presence of blast cells. As a result, all treatment including the disatinib was suspended and the patient’s medical staff acknowledged failure in treating her cancer. It was charted by the patient’s hematologist/oncologist that the patient ‘suffers from terminal malignant disease. She has been treated to the limits of available therapy… no further active intervention will be undertaken’. She was placed in palliative home care and told to prepare for her disease to overwhelm her body and from which she would suffer a stroke within the next 2 months.
Cannabinoid Treatment
After this, disease progression was observed with rising counts of blast cells. The patient was receiving frequent blood transfusions and platelets during this period. Through research conducted by the patient’s family, it was observed, in a particular paper by Guzman [1] published in Nature Reviews Cancer, that cannabinoids have been shown to inhibit the growth of tumor cells in culture and in animal models by modulating key cell-signaling pathways. Cannabinoids are usually well tolerated and do not produce the generalized toxic effects of conventional chemotherapies. The family found promise in an organization known as Phoenix Tears, led by Rick Simpson who had treated several cancers with hemp oil, an extract from the cannabis plant. Rick worked with the family to help them prepare the extract.
From the 4th to the 20th of February, the patient’s blast cell count had risen from 51,490 to 194,000. The first dose of cannabinoid resin, also referred to as ‘hemp oil’, was administered orally (1 drop about the size of half a grain of rice) at 6:30 a.m. on the 21st February 2009 (day 0 in fig. 1). A 2-ounce Cannabis indica strain (known as ‘Chronic Strain’) was used to extract 7.5 ml of hemp oil using 1.2 liters of 99%-isopropyl-alcohol solution, which was boiled off in a rice cooker. Immediately after the dosing, the patient attempted to vomit; nausea had been observed previously and is common with this condition. To deal with the bitter taste and viscous nature of the hemp oil, it was suspended in honey, a known natural digestive aid, and then administered to the patient in daily doses. The objective was to quickly increase the frequency and amount of the dose and to hopefully build up the patient’s tolerance to cannabinoid resin (refer to fig. 1). The patient was observed to have periods of panic early on during administration of the hemp oil, along with increased appetite and fatigue.
Fig 1: Blast cell counts, days 0-15: Chronic Strain.
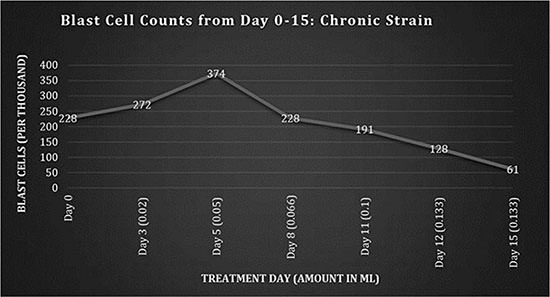
The blast cell count reached a peak of 374,000 on the 25th February 2009 (day 5), followed by a decrease, which correlated with the increasing dose. The daily dosing is the amount administered per dose; the doses were initially given once per day up to a total of 3 times per day by day 15, and were continued with the same average frequency throughout the treatment. A decreased use of morphine for pain, an increase in euphoria symptoms, a disoriented memory and an increase in alertness were observed; these are typical with cannabinoid use.
After day 15, the original Chronic Strain had been consumed and administration of a new strain (referred to as Hemp Oil #2) was started. This was obtained by the family from an outside source. It was noted that administering the same dose yielded a decreased response in terms of the side effects of euphoria and appetite, and the patient suffered more nausea with this hemp oil. The blast cells began to increase, demonstrated in figure 2.
Fig 2: Blast cell counts, days 18-39: Hemp Oil #2.
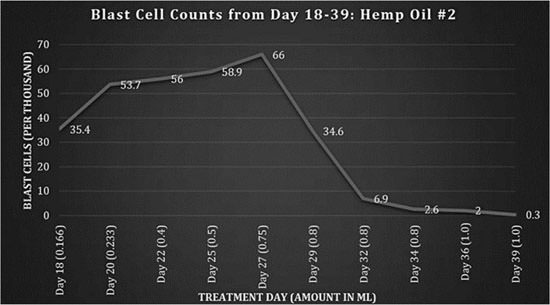
There is a wide amount of variance in cannabinoid concentration amongst different strains and even in the same strain with changes in growing conditions. The amount of each dose was increased to match the response of the blast cells that had been declining previously (fig. 1). After day 27, there was another peak blast cell count of 66,000 followed by a rapid decrease. There were elevated levels of urate present in the blood with corresponding joint pain; it was established that this was caused by tumor lysis syndrome of the blast cells. Allopurinol was administered.
On the 1st April 2009 (day 41), an infected central line with tunnel infection was noted on a blood test and the patient was admitted with a heavy antibiotic regimen of intravenous tazocin, gentamicin and vancomycin. On day 43, a new batch of hemp oil from an Afghan/Thai strain (referred to as Hemp Oil #3) prepared by the family was administered. A stronger psychosomatic response and increased fatigue were observed, so dosing was adjusted to 0.5 ml, shown in figure 3. Due to hospital restrictions, dosing was limited to twice a day.
Fig 3: Blast cell counts, days 44-49: Hemp Oil #3.
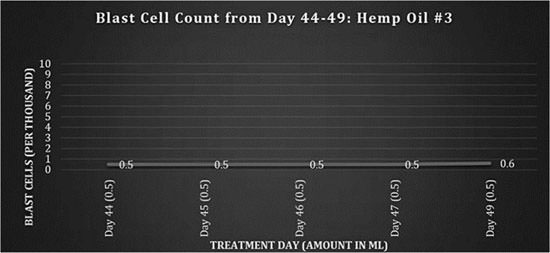
A new batch of hemp oil was obtained by the family from an outside source and the dosing regimen continued twice a day, shown in figure 4.
Fig 4: Blast cell counts, days 50-67: Hemp Oil #4.
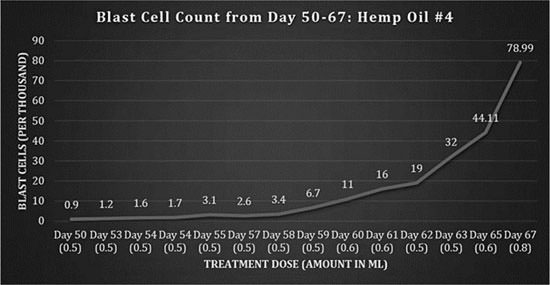
After returning home from the hospital on the 11th April (day 51), the patient suffered from intense nausea, an inability to eat and overall weakness. On the 13th April, the patient was readmitted to the SickKids Hospital and was treated for refeeding syndrome. This was the outcome of stopping total parenteral nutrition too quickly and causing shock to the patient’s body while she was being treated for the infection. The dosing regimen was intermittent until day 59, remaining at 1-2 doses per day of 0.5 ml. As the blast cells began to increase and the patient’s appetite increased, the dosing frequency was again increased to 3 times per day starting on day 62, and the amount administered was increased from day 65.
On day 68, a new batch of medicine was obtained by the family from an outside source (referred to as Hemp Oil #5), shown in figure 5.
Fig 5: Blast cell counts, days 69-78: Hemp Oil #5.
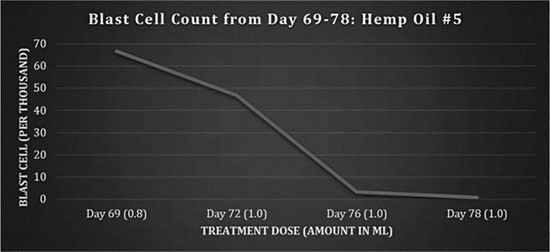
Dosing was maintained 3 times a day at 1.0 ml. On day 78, the patient had stomach pain in the morning and was admitted to hospital. Upon X-ray, it was noted that gastrointestinal bleeding had occurred. The patient was under a DNR order and ultimately passed away due to the bowel perforation. A prior history of pancolitis documented by CT scan in March 2009 pointed to neutropenic colitis with perforation as the cause of death. Furthermore, prior to starting on the hemp oil treatment, the patient had been extremely ill, severely underweight and had endured numerous sessions of chemotherapy and radiation therapy in the course of 34 months.
As reported by Hematology/Oncology at SickKids:
‘At admission her total WBC was 1.4, hemoglobin was 82, platelet count 8,000. She was profoundly neutropenic… a prior history of pancolitis documented by CT scan in March 2009 was neutropenic colitis with perforation… her abdomen was distended and obviously had some signs of diffuse peritonitis. The abdomen X-ray was in favour a perforation…she passed away at 10:05 in the present (sic) of family…’.
Discussion
Figure 6 is a summary of dose response to all the batches of hemp oil administered over a total of 78 days.
Fig 6: Response to hemp oil treatment over 78 days.
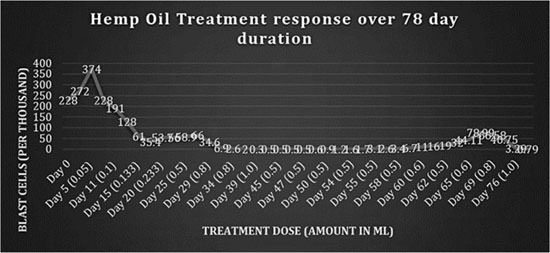
The results shown here cannot be attributed to the phenomenon of ‘spontaneous remission’ because a dose response curve was achieved. Three factors, namely frequency of dosing, amount given (therapeutic dosing) and the potency of the cannabis strains, were critical in determining response and disease control. By viewing figure 6, it can be seen that introducing strains that were less potent, dosing at intervals >8 h and suboptimal therapeutic dosing consistently showed increases in the leukemic blast cell count. It could not be determined which cannabinoid profiles constituted a ‘potent’ cannabis strain because the resin was not analyzed. Research is needed to determine the profile and ratios of cannabinoids within the strains that exhibit antileukemic properties.
These results cannot be explained by any other therapies, as the child was under palliative care and was solely on cannabinoid treatment when the response was documented by the SickKids Hospital. The toxicology reports ruled out chemotherapeutic agents, and only showed her to be positive for THC (tetrahydrocannabinol) when she had ‘a recent massive decrease of WBC from 350,000 to 0.3′ inducing tumor lysis syndrome, as reported by the primary hematologist/oncologist at the SickKids Hospital.
This therapy has to be viewed as polytherapy, as many cannabinoids within the resinous extract have demonstrated targeted, antiproliferative, proapoptotic and antiangiogenic properties. This also needs to be explored further, as there is potential that cannabinoids might show selectivity when attacking cancer cells, thereby reducing the widespread cytotoxic effects of conventional chemotherapeutic agents. It must be noted that where our most advanced chemotherapeutic agents had failed to control the blast counts and had devastating side effects that ultimately resulted in the death of the patient, the cannabinoid therapy had no toxic side effects and only psychosomatic properties, with an increase in the patient’s vitality.
The nontoxic side effects associated with cannabis may be minimized by slowly titrating the dosing regimen upwards, building up the patient’s tolerance. The possibility of bypassing the psychoactive properties also exists, by administering nonpsychoactive cannabinoids such as cannabidiol that have demonstrated antiproliferative properties. Furthermore, future therapies could examine the possibility of upregulating a patient’s endogenous cannabinoids to help combat leukemic cells. It goes without saying that much more research and, even more importantly, phase clinical trials need to be implemented to determine the benefits of such therapies. Laboratory analysis is critical to figure out the constituents/profiles/ratios of the vast cannabis strains that show the most favored properties for exerting possible anticancer effects. Despite the nonstandardization of the medicines, the dose was readily titrated according to the biological response of the patient and produced a potentially life-saving response, namely, the drop in the leukemic blast cell count.
There has been an abundance of research exhibiting the cytotoxic effects of cannabinoids on leukemic cell lines in the form of in vitro and in vivo studies [1,2,3,4]. An oncology and hematology journal, Blood, has published numerous papers [2] over the years constructing the biochemical pathway to be elicited by the anticancer properties of cannabinoids. Our goal, upon examination of this significant case study which demonstrated complete disease control and a dose response curve, is to invest effort in and to focus on research and development to advance this therapy. An emphasis needs to be placed on determining the correct cannabinoid ratios for different types of cancer, the best method of administration, quality control and standardization of the cannabis strains and their growing conditions as well as therapeutic dosing ranges for various cancers contingent on staging and ages. Toxicity profiles favor therapies deriving from cannabis because toxicity within the body is greatly reduced and the devastating side effects of chemoradiation (i.e. secondary cancers or death) can be eliminated. It is unfortunate that this therapy does come with some unwanted psychosomatic properties; however, these might be eliminated by target therapies of nonpsychoactive cannabinoids such as cannabidiol which has garnered much attention as being a potent anti-inflammatory and possible antileukemic and anticancer agent. It is acknowledged that significant research needs to be conducted to reproduce these results and that in vitro studies cannot always be reproduced in clinical trials and the human physiological microenvironment. However, the numerous research studies and this particular clinical case are powerful enough to warrant implementing clinical trials to determine dose ranges, cannabinoid profiles and ratios, the methods of administration that produce the most efficacious therapeutic responses and the reproducibility of the results. It is tempting to speculate that, with integration of this care in a setting of full medical and laboratory support, a better outcome may indeed be achieved in the future.
References
- Guzman M: Cannabinoids: potential anti-cancer agent. Nat Rev Cancer 2003;3:745-755.
- Powles T, Poele RT, Shamash J, Chaplin T, Propper D, Joel S, Oliver T, Liu WM: Cannabis-induced cytotoxicity in leukemic cell lines: the role of the cannabinoid receptors and the MAPK pathway. Blood 2005;105:1214-1221.
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M: Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol 2006;70:897-908.
- Murison G, Chubb C, Maeda S, Gemmell MA, Huberman E: Cannabinoids induce incomplete maturation of cultured human leukemia cells. Proc Natl Acad Sci 1987;84:5414-5418.
Author Contacts
Yadvinder Singh
44 Don Minaker Drive
Brampton, ON, L6P1R3 (Canada)
E-Mail [email protected]










