Abstract
Background
Cannabidiol (CBD) is one of the main phytocannabinoids found in Cannabis sativa. In contrast to Δ9-tetrahydrocannabinol, it has a low affinity for cannabinoid receptors CB1 and CB2, thereby it does not induce significant psychoactive effects. However, CBD may interact with other receptors, including peroxisome proliferator-activated receptor gamma (PPARγ). CBD is a PPARγ agonist and changes its expression. There is considerable evidence that CBD’s effects are mediated by its interaction with PPARγ. So, we reviewed studies related to the interaction of CBD and PPARγ.
Methods
In this comprehensive literature review, the term ‘cannabidiol’ was used in combination with the following keywords including ‘PPARγ’, ‘Alzheimer’s disease’, ‘Parkinson’s disease’, ‘seizure’, ‘multiple sclerosis’, ‘immune system’, ‘cardiovascular system’, ‘cancer’, and ‘adipogenesis’. PubMed, Web of Science, and Google Scholar were searched until December 20, 2022. A total of 78 articles were used for the reviewing process.
Results
CBD, via activation of PPARγ, promotes significant pharmacological effects. The present review shows that the effects of CBD on Alzheimer’s disease and memory, Parkinson’s disease and movement disorders, multiple sclerosis, anxiety and depression, cardiovascular system, immune system, cancer, and adipogenesis are mediated, at least in part, via PPARγ.
Conclusion
CBD not only activates PPARγ but also affects its expression in the body. It was suggested that the late effects of CBD are mediated via PPARγ activation. We suggested that CBD’s chemical structure is a good backbone for developing new dual agonists. Combining it with other chemicals enhances their biological effectiveness while reducing their dosage. The present study indicated that PPARγ is a key target for CBD, and its activation by CBD should be considered in all future studies.
Graphical abstract
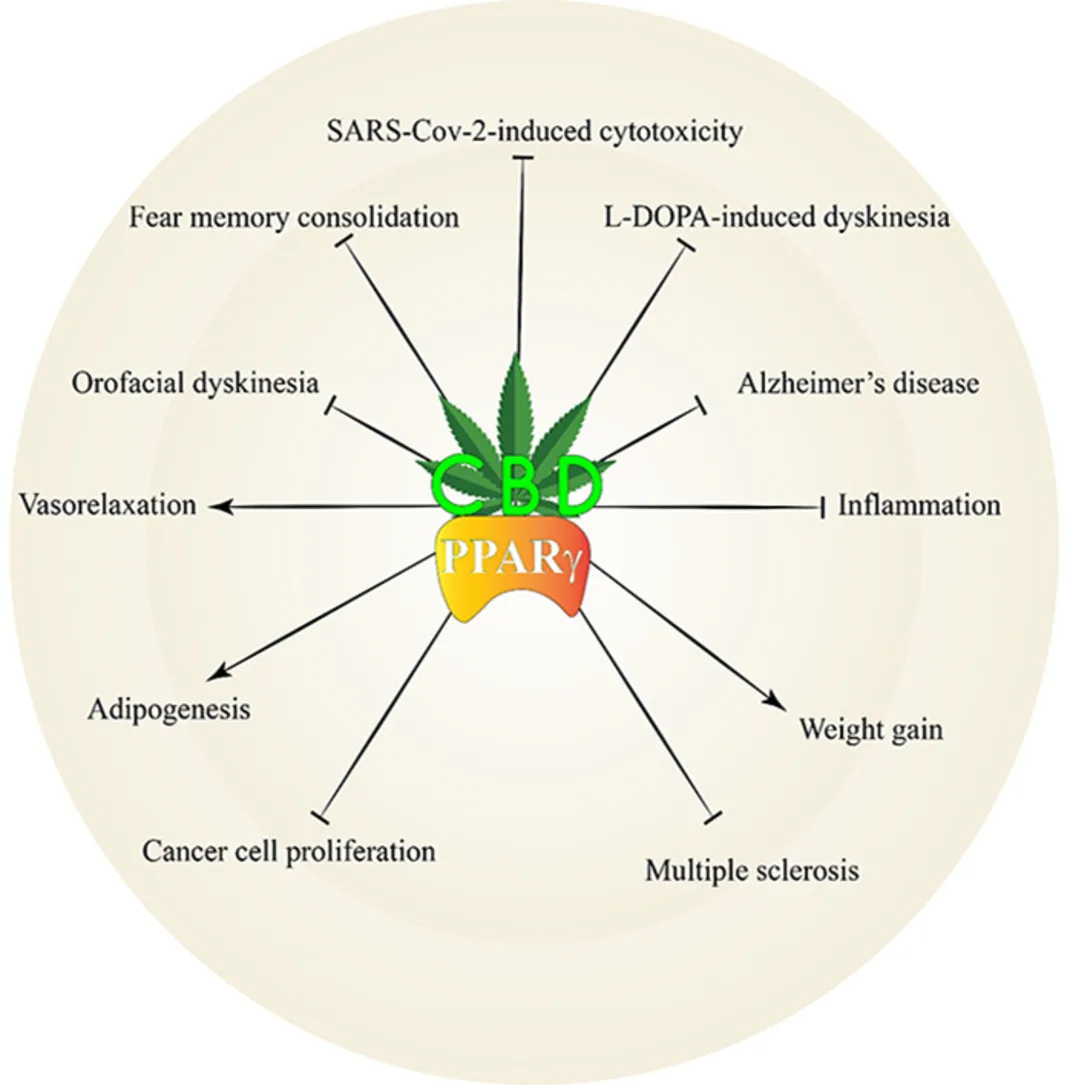
Introduction
Cannabis sativa is a medicinal plant with many active compounds, including Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) (Martínez et al., 2020). Δ9-THC is a phytocannabinoid with psychoactive properties, while CBD is devoid of such properties. However, CBD has been used in the treatment of a wide range of diseases and disorders, such as psychosis, depression, epilepsy, anxiety, addiction, multiple sclerosis, cardiovascular and inflammatory diseases, rheumatoid arthritis, emesis, Parkinson’s, Alzheimer’s, and Huntington’s diseases (Melas et al., 2021; Premoli et al., 2019; Rock et al., 2012; Rudroff and Sosnoff, 2018; Stanley et al., 2013; Verrico et al., 2020). This wide range of applications suggests diverse cellular targets for CBD. Indeed, CBD has a rich pharmacology with numerous biological targets. After FDA approval of Epidiolex® for the treatment of Dravet and Lennox-Gastaut syndromes (Devinsky et al., 2017; Thiele et al., 2018) and the recent legalization of commercial hemp, CBD has gained much more attention. Its applications in the treatment of anxiety (Berger et al., 2022), post-traumatic stress disorder (PTSD) (Das et al., 2013), psychosis (Devinsky et al., 2014), cognitive dysfunction (McCartney et al., 2022), and dementia (Kłosińska and Leszko, 2022) have been reported with positive results.
It was reported that consumption of a marijuana cigarette (1 g) elevates CBD concentration up to 0.056 μg/ml in the serum (Pacifici et al., 2020). Cannabinoids and endocannabinoids (e.g., anandamide and 2-arachidonoylglycerol) act primarily on two cannabinoid receptors, the CB1 and CB2 receptors (Hajizadeh Moghaddam et al., 2013). Although CBD has little or no binding affinity for these targets (Navarro et al., 2018). So, the discovery of biological targets other than CB1 and CB2 has been pursued in many recent studies. CBD binds various receptors, including the orphan G protein-coupled receptors; GPR3, GPR6, GPR12, and GPR55 (Laun et al., 2019; Whyte et al., 2009), α2 adrenergic, (Cascio et al., 2010), dopamine D2 (Seeman, 2016), γ-aminobutyric acid type A (GABAA) (Bakas et al., 2017), adenosine A2A (Carrier et al., 2006), glycine (Ahrens et al., 2009), serotonin 5-HT1A (Galaj et al., 2020), and μ- and δ-opioid receptors (Kathmann et al., 2006). It also binds T-type (Ross et al., 2008) and l-type voltage-regulated Ca2+ channels (Isaev et al., 2022), and peroxisome proliferator-activated receptor gamma (PPARγ) (Mlost et al., 2021), and has significant modulatory effects on various transient potential (TRP) channels (Etemad et al., 2022).
PPARγ belongs to a family of nuclear receptors having three subtypes: PPARα, PPARβ/δ, and PPARγ. They participate in several cellular functions, including respiration, metabolism, differentiation, and development (Mirza et al., 2019) and serve as the main regulators of energy homeostasis and glucose and lipid metabolism. PPARγ has two distinct isoforms designated as PPARγ1 and PPARγ2. PPARγ1 is expressed in almost all tissues, while PPARγ2 is expressed almost exclusively in the adipose tissue (Ahmadian et al., 2013). Due to its different structure, PPARγ2 has 5- to 10-fold ligand-independent activation compared to PPARγ1, with an important role in effective adipogenesis (Wu et al., 2020). Several endogenous ligands for PPARγ have been identified, mainly belonging to unsaturated fatty acids, including petroselinic acid, arachidonic acid, linolenic acid, and linoleic acid (Kliewer et al., 1997). PPARγ ligands promote PPARγ heterodimer with another nuclear receptor, the retinoid X receptor (RXR). The functions of PPARγ get altered by the binding ligand shape and the presence of coactivator or corepressor proteins. When no ligand is present, PPARγ and RXR heterodimer binds with the corepressor and suppress gene transcription (Privalsky, 2004). In contrast, specific ligands lead to recruitment of the coactivator and release the corepressor that induces activation of the basal transcriptional machinery. So, PPARγ changes the expression of various genes implicated in glucose homeostasis, insulin release, lipid metabolism, inflammation, and immunity (Fig. 1) (Ahmadian et al., 2013). PPARγ activates nuclear erythroid 2-related factor (Nrf2), which provokes the expression of major antioxidant proteins such as glutathione S-transferase (GST), catalase (CAT), heme-oxygenase-1 (HO-1), and manganese-dependent superoxide dismutase (Mn-SOD) (Hussein et al., 2019; Nguyen et al., 2009). PPARγ also inhibits the transcriptional activities of several transcription factors including activator protein 1 (AP-1), signal transducer and activator of transcription (STAT), nuclear factor-kappa B (NF-kB), and cell signaling proteins, including mitogen-activated protein (MAP) kinases (Carvalho et al., 2021). NF-kB has a crucial role in the activation of various pro-inflammatory gene expressions such as tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), interleukin-1 (IL-1), IL-6, and IL-12 (Liu et al., 2017). PPARγ agonists (thiazolidinediones) such as pioglitazone and rosiglitazone are used for treating type II diabetes. However, they have been used in the treatment of various diseases such as cancer (Yee et al., 2007), Alzheimer’s disease (Sato et al., 2011), depression (Kemp et al., 2014), and rheumatoid arthritis (Ormseth et al., 2013). This wide range of clinical applications is in accordance with multiple PPARγ cellular targets that were mentioned previously.
However, thiazolidinediones may exacerbate congestive heart failure in some patients (Chaggar et al., 2009). These drugs may increase the risk of bladder cancer and bone fractures (Murphy and Rodgers, 2007; Tseng, 2014). The underlying mechanism is not well established, but it may result from activation of PPARγ. PPARγ activation alters tumor growth and progression in non-adipose cells. Its activation in bone creates an imbalance in bone remodeling, including changes in bone marrow structure and function. Thiazolidinediones may also impair bone function by reducing estrogen synthesis (Murphy and Rodgers, 2007).
CBD is an agonist of the PPARγ (Granja et al., 2012) and has little or no affinity for PPARα (D’Aniello et al., 2019). Likewise, some cannabinoids, such as THC (O’Sullivan et al., 2005) and the endocannabinoids 2-arachidonoylglycerol and anandamide, have affinities for PPARγ (Gasperi et al., 2007). CBD not only binds PPARγ but also changes its expression (Hegde et al., 2015; O’Sullivan et al., 2009). Considering the safety and tolerability of CBD in clinical practice (McCartney et al., 2022; O’Brien et al., 2022) and its growing consumption (Rapin et al., 2021), we have to expand our knowledge regarding the biological targets of CBD. Considerable evidence shows that PPARγ is a key target for CBD. Indeed, many effects of CBD can be prevented by PPARγ antagonists. Based on these findings, for the first time, we aimed to review the studies related to the interaction of CBD and PPARγ and discuss the importance of such interaction in pre-clinical and clinical studies.
Section Snippets
Search Strategy
In this comprehensive literature review, the term ‘cannabidiol’ was used in combination with the following keywords including ‘PPARγ’, ‘Alzheimer’s disease’, ‘Parkinson’s disease’, ‘seizure’, ‘multiple sclerosis’, ‘immune system’, ‘cardiovascular system’, ‘cancer’, ‘adipogenesis’. PubMed, Web of Science, Scopus, and Google Scholar were used as electronic databases. No time limitation (up to December 20, 2022) was considered in this review. The search strategy used for the present article…
CBD and PPARγ interaction in the cardiovascular system
CBD has been reported as a vasorelaxant agent that reduces mean arterial blood pressure without affecting the heart rate (Walsh et al., 2010). The vasorelaxant effect of CBD on human mesenteric arteries has been reported (Stanley et al., 2015). The respective molecular basis was investigated in the isolated rat small mesenteric arteries (sMAs) and human pulmonary arteries (hPAs) (Baranowska-Kuczko et al., 2020). The results showed that CBD promoted a full concentration- and time-dependent…
CBD and PPARγ interaction in the immune system
PPARγ expression may alter under inflammatory conditions (Wahli and Michalik, 2012). It inhibits gene expression of various pro-inflammatory mediators, including NO, IL-1β, IL-6, TNF-α, iNOS, and COX-2 via NF-κB inhibition (Liu et al., 2017). In addition, PPARγ promotes macrophage differentiation to the anti-inflammatory M2 phenotype and suppresses the pro-inflammatory M1 phenotype (Bouhlel et al., 2007). PPARγ receptor has been associated with cell apoptosis, proliferation, and reduction of
CBD and PPARγ interaction in viral infections
In recent years, CBD has received more attention as an antiviral agent. It has an antiviral effect on Kaposi’s sarcoma-associated herpes virus (Maor et al., 2012) and hepatitis C virus, but not on hepatitis B virus (Lowe et al., 2017). CBD’s anti-inflammatory and analgesic properties make it plausible for use in oral and genital herpes, shingles, and Ebola (Mabou Tagne et al., 2020). CBD also potentiated the antiviral effects of terpenes against human Coronavirus E229 (Chatow et al., 2021). A…
CBD and PPARγ interaction in cancer
Successful treatment of cancer remains one of the major problems of human health. Many studies show that CBD has beneficial effects on cancer cells both in vitro and in vivo. CBD exhibited anti-proliferative properties against colon, breast, cervix, glioma, prostate, ovary, leukemia, and thyroid cancer cells (Massi et al., 2013). The main mechanisms underlying the anticancer effects of CBD are inhibition of intercellular adhesion molecule-1 (ICAM-1)-dependent cell invasion, reduction in…
CBD and PPARγ interaction in adipogenesis
PPARγ has a crucial role in white adipose tissue adipogenesis and is considered a “master regulator” of adipogenesis (Wafer et al., 2017). Mutations within the PPARγ gene have been associated with severe lipodystrophy, insulin resistance, and diabetes in humans (Agostini et al., 2006). Considering the crucial role of PPARγ in adipogenesis, the effect of CBD on adipogenesis in human and mouse multipotent MSCs was assessed. The results revealed that CBD promoted the differentiation of MSCs and…
Conclusion and perspectives
PPARγ receptors are attractive drug targets as they modulate various physiological processes. They may offer a promising target for treating diseases such as AD due to their variable expression during selected diseases (de la Monte and Wands, 2006). PPARγ agonists are a class of anti-diabetic drugs whose applications are beyond their glucose-lowering effects; breast cancer (Yee et al., 2007), rheumatoid arthritis (Ormseth et al., 2013), high blood pressure (Piché et al., 2018), endothelial…
[ View Full Text ]
